Introduction: Non-Follicular Low-Grade B-Cell Lymphomas (LGBCL) are a subset of lymphomas that share overlapping clinical and histologic features. While most cases of LGBCL have an indolent course, some experience aggressive disease with no definitive biomarkers to identify aggressive cases at diagnosis. Significant advances have been made in the molecular classification of other lymphomas, yet molecular profiling and identification of mechanisms driving aggressive disease in LGBCL remain an unmet need. We previously used a multi-omic approach to characterize the molecular and immune landscapes of LGBCL tumors and identified a transcriptomic signature associated with cell proliferation and inferior overall survival (OS) in two independent cohorts (HR 7.82; 95% CI 2.4-25.4; p < 0.001 & HR 10.07; 95% CI 2.00-50.61; p = 0.005) and cases that transformed to DLBCL (Hopper et al., 2023). Here, we identify the oncoprotein DEK as a novel transcriptional regulator of this signature and use a functional genomic approach to further interrogate the role of DEK in B-cell lymphoma.
Methods: Tumors from 64 newly-diagnosed/untreated LGBCL cases enrolled in the University of Iowa/Mayo Clinic Molecular Epidemiology Resource were included in this study (SMZL = 48, NMZL = 6, LPL = 5, B-NOS = 3, EMZL = 2). Tumor RNA-seq and paired tumor-normal WES were used to assess DEK expression and identify copy number alterations (CNAs), respectively. DEK deficient (KO) cell line models were generated using CRIPSR/Cas9. DEK expression was assessed by western blot (WB) and immunofluorescence (IF) using standard techniques. Proliferation was assessed by MTT assay.
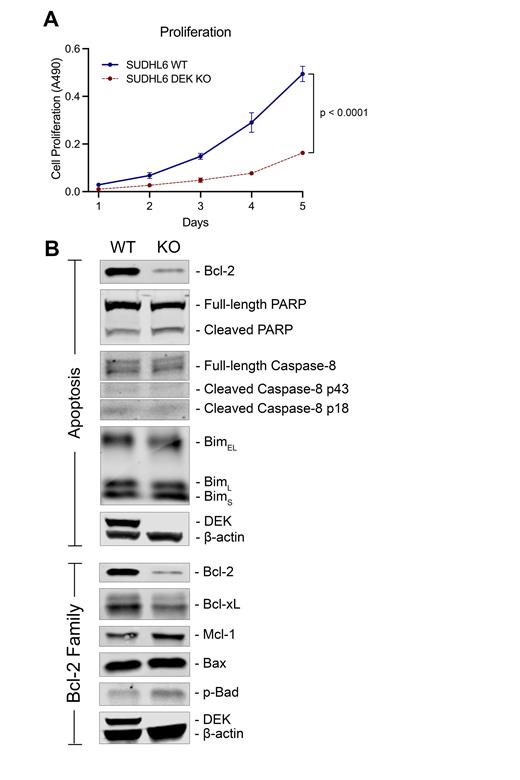
Results: To identify transcriptional regulators of our previously identified signature, we used the tool MAGIC to mine publicly available ChIP-seq data and identified DEK, an oncoprotein associated with tumorigenesis and poor prognosis in several cancers, as a key regulator of genes in our signature (p = 0.008). RNA-seq indicated that high levels of DEK expression associated with inferior OS in LGBCL (HR 2.99; 95% CI 1.21-7.36; p = 0.02). Additionally, we identified DEK as a target of copy number gains/amps in LGBCL (7% of cases), which corresponded to significantly higher DEK expression (p = 0.04). The association of DEK with aggressive lymphoma was confirmed when we assessed its expression in public cell line RNA-seq data and observed the highest expression in lines derived from aggressive lymphomas, which we further validated at the protein level in a panel of B-cell lymphoma cell lines by WB and IF confocal microscopy. Additionally, we detected nuclear localization of DEK, consistent with its role as a transcriptional regulator. As a model of DEK overexpression for functional characterization, we used the SUDHL6 lymphoma cell line, which has an amplification of DEK at 6p22.3 and expresses high levels of DEK protein. Using CRISPR technology, we generated DEK deficient SUDHL6 and tested the impact of loss of DEK expression. DEK KO cells proliferated significantly slower than WT SUDHL6 cells in vitro (p < 0.0001)(Panel A), indicating DEK plays a role in regulating proliferative capacity in lymphoma. To identify mechanisms responsible for reduced proliferation in DEK KOs, we assessed baseline protein expression levels of apoptosis proteins in WT versus KO cells. We found near complete abrogation of anti-apoptotic protein Bcl-2 and reduction of Bcl-xL expression in DEK KO cells (Panel B). These results indicate DEK may contribute to tumor proliferative capacity by regulating susceptibility to apoptosis.
Conclusion: DEK has been implicated in regulation of gene expression, but its precise role has remained elusive, with little known about its role in lymphoma. Using an integrative molecular and functional genomics approach, we identified DEK as a novel oncoprotein in LGBCL that is upregulated in cases with inferior clinical outcome and is regulated by copy number gains/amps. DEK plays a role in regulating tumor proliferative capacity by altering susceptibility to apoptosis through regulation of Bcl-2 and Bcl-xL. Collectively, this data suggests high-risk LGBCL tumors may enrich for cells with DEK overexpression or CNAs as a mechanism driving oncogene addiction, highlighting DEK, and its downstream targets, as potential therapeutic targets in a subset of high-risk LGBCL.
Disclosures
Wenzl:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Habermann:sorrento: Research Funding; BMS: Research Funding; Genentech: Research Funding. Rimsza:Roche: Other: Consulting; NanoString: Other: Licensed intellectual property. Witzig:Salarius Pharma: Membership on an entity's Board of Directors or advisory committees; ADC: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Kura Oncology: Research Funding. Ansell:ADC Therapeutics, Affimed, Bristol-Myers Squibb Company, Pfizer Inc, Regeneron Pharmaceuticals Inc, Seagen Inc, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research. Cerhan:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Genmab: Research Funding; Protagonist: Other: Safety Monitoring Committee; Genentech: Research Funding. Novak:Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal